
Note that the total number of protons and electrons can be found using the same formula because the total number of protons are always equal to the total number of electrons and the sum of protons and neutrons constitute the mass number of an element. Therefore, total number of neutrons present in potassium element is 20 Where, m is the mass number of an element. Now, by using the formula to calculate the number of neutrons in an atom that is, Together, the number of protons and the number of neutrons determine an element’s mass number: mass number protons + neutrons. Thus, the protons present in the atom is 19. We know that the number of protons is always equal to the number of electrons in any atom and also the total number of protons present is given by their atomic number. It provides atomic mass, mass excess, nuclear binding energy, nucleon separation energies, Q-values, and nucleon residual interaction parameters for atomic. Potassium is having the atomic symbol$P$and according to the data given the atomic number is 19 and the mass number is 39. An atom of potassium with 20 neutrons would have a mass number of 39 and thus be an atom of the potassium-39 isotope. Click the card to flip Argon has a greater proportion of heavier isotopes with more neutrons than potassium. Let us now calculate the number of neutrons present in the potassium as per the data given. Explain why the relative atomic mass of argon is greater than the relative atomic mass of potassium, even though the atomic number of potassium is greater than the atomic number of argon. Atoms are the smallest species about 100 picometers in size and are composed of protons that are positively charged and neutrons that are the neutral atoms and the electrons that are negatively charged revolve around it.
In the lower classes of chemistry, we have studied about the concepts of general chemistry which deals with the fact about the structure of atom and its constituent parts that is nucleus Hint: The answer here is based on the facts about the general concepts of chemistry which says that number of protons is equal to electrons and the number of neutrons can be determined using the formula,$$ When an organism dies, it stops taking in carbon-14, so the ratio of carbon-14 to carbon-12 in its remains, such as fossilized bones, will decline as carbon-14 decays gradually to nitrogen-14 2 ^2 2 squared. As animals eat the plants, or eat other animals that ate plants, the concentrations of carbon-14 in their bodies will also match the atmospheric concentration. All other alkali metals immediately follow noble gases (they have. The alkali metal has a smaller atomic weight than the noble gas and appears before the noble gas in the sequence of atomic weights. Potassium, atomic number 19 has an atomic mass of 39.10 u. As plants pull carbon dioxide from the air to make sugars, the relative amount of carbon-14 in their tissues will be equal to the concentration of carbon-14 in the atmosphere. Argon, atomic number of 18 has atomic mass of 39.95 u. The atomic number of each element increases by one, reading from left to right. Period A horizontal row in the periodic table. Determine the number of protons, neutrons, and electrons in an atom. Members of a group typically have similar properties and electron configurations in their outer shell. Elizabeth Gordon Furman University Learning Outcomes Define atomic and mass numbers.
Also see Atomic number of different elements.
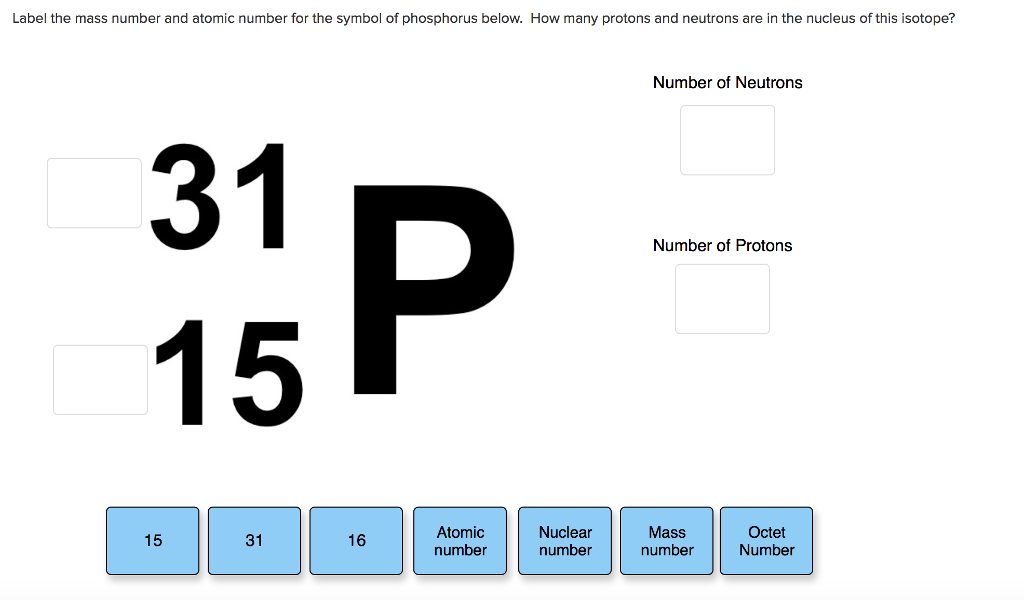
The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. Potassium is a chemical element of the periodic table with chemical symbol K and atomic number 19 with an atomic weight of 39.0983 u and is classed as. These forms of carbon are found in the atmosphere in relatively constant proportions, with carbon-12 as the major form at about 99%, carbon-13 as a minor form at about 1%, and carbon-14 present only in tiny amounts 1 ^1 1 start superscript, 1, end superscript. Potassium 19 39.098 Glossary Group A vertical column in the periodic table. potassium chloride + silver nitrate silver chloride (s) + potassium nitrate. Potassium is a chemical element with atomic number 19 which means there are 19 protons in its nucleus. A property closely related to an atom’s mass number is its atomic mass. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from the mass number. For example, carbon is normally present in the atmosphere in the form of gases like carbon dioxide, and it exists in three isotopic forms: carbon-12 and carbon-13, which are stable, and carbon-14, which is radioactive. Together, the number of protons and the number of neutrons determine an element’s mass number: mass number protons + neutrons.


 0 kommentar(er)
0 kommentar(er)
